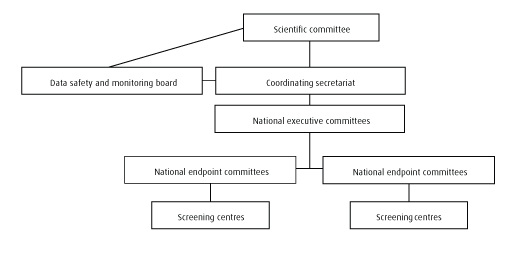
Scientific committee (SC)
Definiton: The SC is the head group in the internal NordICC hierarchy. It consists of at least one member from each of the participating countries and the coordinating secretariat.
Tasks and responsibilities: Overall responsibility and decision authority for the trial in general, including aspects of management, screening, quality control, endpoint observation and publication activity. Reviews and summarizes reports on quality and adverse events from the secretariat and sends information to the DSMB each month during the screening period.
National executive committees (NEC)
Definiton: The national NEC’s are the coordinating groups in the participating countries. At least one member should be from each of the national screening centres. Unless otherwise decided by the National Executive Committee, the chair person should be its representative in the Scientific Committee.
Tasks and responsibilities: Overall responsibility for the national screening centres and the management of the trial in the respective country. Reports to the SC every month.
Endpoint committee (EC)
The EC group is responsible for standardisation of classification of causes of death (extraction forms for uniform classification of deaths) or other endpoints. Due to language problems during revision, there should be one committee in each country, suggestively consisting of one pathologist, one oncologist and one surgeon. The abstraction form should be identical in all participating centres.
Coordinating secretariat (Norway, Oslo)
The Oslo secretariat manages the trial together with the national screening sites and is responsible for data collection from all screening centres. The NordICC main database is situated here. The secretariat sets up and manages the NordICC trial databases, including tracking of data and statistical service. The secretariat should have a continuous knowledge on quality issues and adverse events and report to the scientific committee every month.
Data safety and monitoring board (DSMB)
DSMB is an external group; their members are not connected to the trial in any way. The DSMB gives advice to the SC on adverse event and endpoint evaluation. The DSMB advices the SC on early trial termination. The group should consist of one cancer epidemiologist, one biostatistician and one gastroenterologist.