The Krauss lab. works towards advanced organoids/OoC models and on methods for interrogating them.
Liver organoids
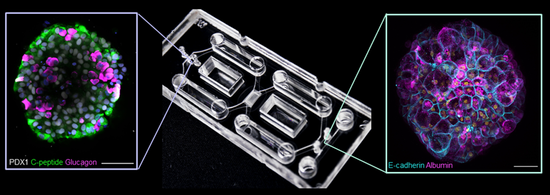
Coming from a developmental biology background, the laboratory works towards an improved structure and functionality of stem cell-derived liver organoids, and hence better physiological representation of the human liver. The liver is shaped by morphogenetic signals from the central vein and the portal triad. Identifying these signals, and applying them for directing organoid development has been a major challenge. Using hESC and hiPSC derived hepatocyte lineages, endothelial lineages and stellate cells we have achieved stable features of zonation in liver organoids and differential response to fibrotic challenges. As a next step, we have integrated liver organoids in a directional flow platform that has been developed in our laboratory. We are now working towards i) integrating the liver organoids with islets (developed in the laboratory of Centre partner Hanne Scholz) ii) including immune components, and ii) increasing the complexity of the liver organoids.
Recent articles: PMID: 37560536, PMID: 37380087.
Major funding: Centre of Excellence RCN grant: 262613.
Novel organ-on-chip formats
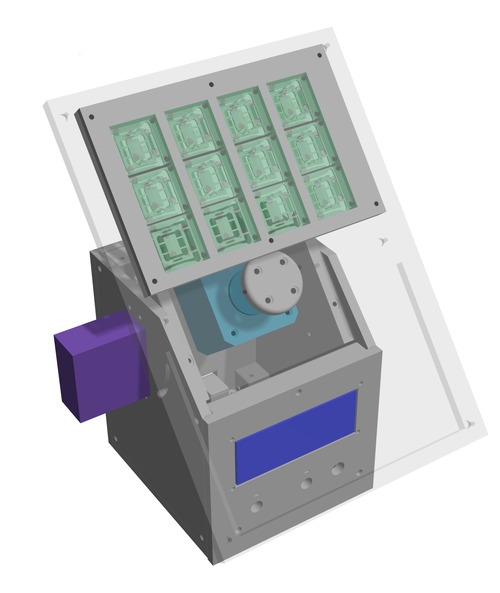
We developed a novel, pump-less directional flow recirculating organ-on-a-chip (rOoC) platform that creates controlled unidirectional gravity-driven flow by a combination of a 3D-tilting system and an optimized microfluidic layout. The platform allows integrating organoids with endothelialized microfluidic channels and components of the immune system. The platform is currently being scaled up in collaboration with Centre partner Nikolaj Gadegaard. In close collaboration with Centre partner Steven Wilson, we have contributed to the development of an organ-in-a-column platform that allows direct on-line LC-MS measurements of metabolites from liver organoids. This has been done by loading a liquid chromatography column with human induced pluripotent stem cell (hiPSC) derived liver organoids, and subsequently coupling this “organ-in-a-column” unit directly with liquid chromatography-mass spectrometry (LC-MS). In a further collaboration with Centre partner Steven Wilson, electromembrane extraction (EME) based on electrophoresis across an oil membrane has been used to segregate selected liver organoid-derived drug metabolites for mass spectrometry (MS)-based measurements.
Recent articles: PMID: 36655405, PMID: 34069506, PMID: 36484723, PMID: 35200386, PMID: 33534551.
Recent reviews: PMID: 37479227.
Patent pending: UK patent application 2110366.8
Major funding: Centre of Excellence grant RCN: 262613, South-Eastern Norway Regional Health Authority: 2021068, The Norwegian Cancer Society: 247751
Gastruloid development
Common organoid technology is based on individual hiPSC derived lineages that are combined into 3D structures. However, despite significant progress in organoid and organ-on- chip technology, it remains challenging to achieve the high physiological and histological complexity of mature organs. A potential alley to reach higher tissue complexity is to develop organs in their naïve embryonic 3D tissue context. Towards this goal we have established a gastruloid sub-group that develops anteriorized mouse and human gastruloids with the aim of reaching organ induction. The group is supported by two Marie Skłodowska Curie fellowships and the recently awarded European Innovation Council (EIC) pathfinder project “supervised morphogenesis”.
Major funding: Centre of Excellence grant RCN: 262613, EIC pathfinder “Supervised Morphogenesis (SUMO), UiO convergence environment (ITOM).
Raman based chemometric imaging on liver organoids
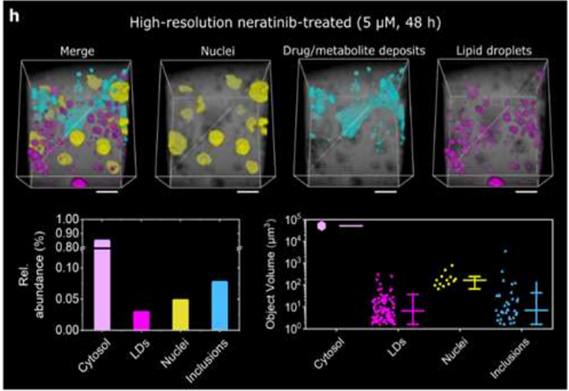
Quantitative chemometric imaging tools for validating the composition of organoids, their functional maturity, disease state and response to therapeutic interventions are of significant interest in the rapidly expanding organoid arena. Raman spectral imaging (RSI) allows high-content, label-free detection of tell-tale biomolecules, but requires reliable quantification of deconvoluted spectra to unfold its full potential. Using qRamanomics, developed in the laboratory of Centre partner Molly Stevens, we first tested liver organoid maturity and variation. We then used the method to identify biomolecular response signatures to a panel of liver altering drugs, probing drug-induced compositional changes in the organoids. We also were able to follow for the first time in situ monitoring of drug metabolism and accumulation in liver organoids.
Recent article: PMID: 37159662.
Major funding: Centre of Excellence grant RCN: 262613.
WNT inhibitor development
The laboratory has a long track record on morphogenetic signals and chemical biology. In particular, we have worked towards developing a WNT/Tankyrase inhibitor. Tankyrase 1 and 2 (TNKS1/2) catalyze post-translational modification by poly-ADP-ribosylation of a plethora of target proteins. In this function, TNKS1/2 also impacts the WNT/β-catenin and Hippo signalling pathways that are involved in numerous human disease conditions. Using various mouse models we have shown efficacy in colon cancer and melanoma tumor models. We are now exploring efficacy in a spectrum of disease models including lung fibrosis and influenza. The work is a collaboration with Centre partner Jo Waaler, the Lari Lehtio laboratory and Symeres Inc.
Recent articles: PMID: 36873622, PMID: 34337362, PMID: 34878777, PMID: 32511917, PMID: 32332858.
Patent pending: WO2019/243822, PCT/GB2021/051714.