 Using the G007-LK tankyrase inhibitor developed in our laboratory, the study unravels a novel molecular mechanism whereby pharmacological inhibition of tankyrase in obesity and diabetes enhances oxidative metabolism and ameliorates lipid disorder. The study shows for the first time that tankyrase associates directly with PGC-1α and that tankyrase inhibition attenuates PARylation of PGC-1α, contributing to increased PGC-1α level in WAT and muscle in db/db mice. PGC-1α upregulation triggers transcriptional reprogramming to increase mitochondrial mass and fatty acid oxidative metabolism in muscle, beiging of WAT, and to augment circulating adiponectin levels. The study highlights pharmacological inhibition of tankyrase as a potential pharmacotherapy for obesity and T2DM. The study was published in the International Journal of Obesity (Nature group, impact factor 5.004)
Using the G007-LK tankyrase inhibitor developed in our laboratory, the study unravels a novel molecular mechanism whereby pharmacological inhibition of tankyrase in obesity and diabetes enhances oxidative metabolism and ameliorates lipid disorder. The study shows for the first time that tankyrase associates directly with PGC-1α and that tankyrase inhibition attenuates PARylation of PGC-1α, contributing to increased PGC-1α level in WAT and muscle in db/db mice. PGC-1α upregulation triggers transcriptional reprogramming to increase mitochondrial mass and fatty acid oxidative metabolism in muscle, beiging of WAT, and to augment circulating adiponectin levels. The study highlights pharmacological inhibition of tankyrase as a potential pharmacotherapy for obesity and T2DM. The study was published in the International Journal of Obesity (Nature group, impact factor 5.004)
Read the article here: https://www.nature.com/articles/s41366-020-0573-z
Abstract:
Objective
Human TNKS, encoding tankyrase 1 (TNKS1), localizes to a susceptibility locus for obesity and type 2 diabetes mellitus (T2DM). Here, we addressed the therapeutic potential of G007-LK, a TNKS-specific inhibitor, for obesity and T2DM.
Methods
We administered G007-LK to diabetic db/db mice and measured the impact on body weight, abdominal adiposity, and serum metabolites. Muscle, liver, and white adipose tissues were analyzed by quantitative RT-PCR and western blotting to determine TNKS inhibition, lipolysis, beiging, adiponectin level, mitochondrial oxidative metabolism and mass, and gluconeogenesis. Protein interaction and PARylation analyses were carried out by immunoprecipitation, pull-down and in situ proximity ligation assays.
Results
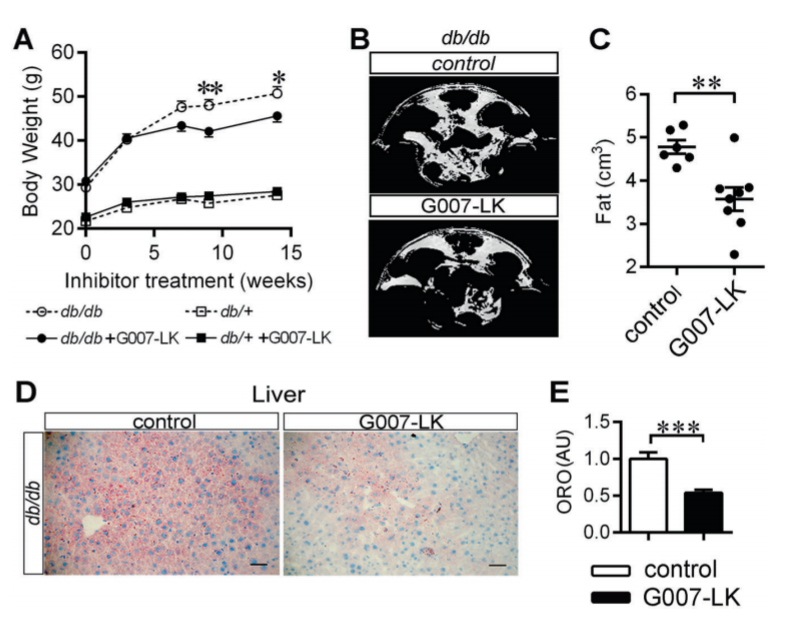
TNKS inhibition reduced body weight gain, abdominal fat content, serum cholesterol levels, steatosis, and proteins associated with lipolysis in diabetic db/db mice. We discovered that TNKS associates with PGC-1α and that TNKS inhibition attenuates PARylation of PGC-1α, contributing to increased PGC-1α level in WAT and muscle in db/db mice. PGC-1α upregulation apparently modulated transcriptional reprogramming to increase mitochondrial mass and fatty acid oxidative metabolism in muscle, beiging of WAT, and raised circulating adiponectin level in db/db mice. This was in sharp contrast to the liver, where TNKS inhibition in db/db mice had no effect on PGC-1α expression, lipid metabolism, or gluconeogenesis.
Conclusion
Our study unravels a novel molecular mechanism whereby pharmacological inhibition of TNKS in obesity and diabetes enhances oxidative metabolism and ameliorates lipid disorder. This happens via tissue-specific PGC-1α-driven transcriptional reprogramming in muscle and WAT, without affecting liver. This highlights inhibition of TNKS as a potential pharmacotherapy for obesity and T2DM.