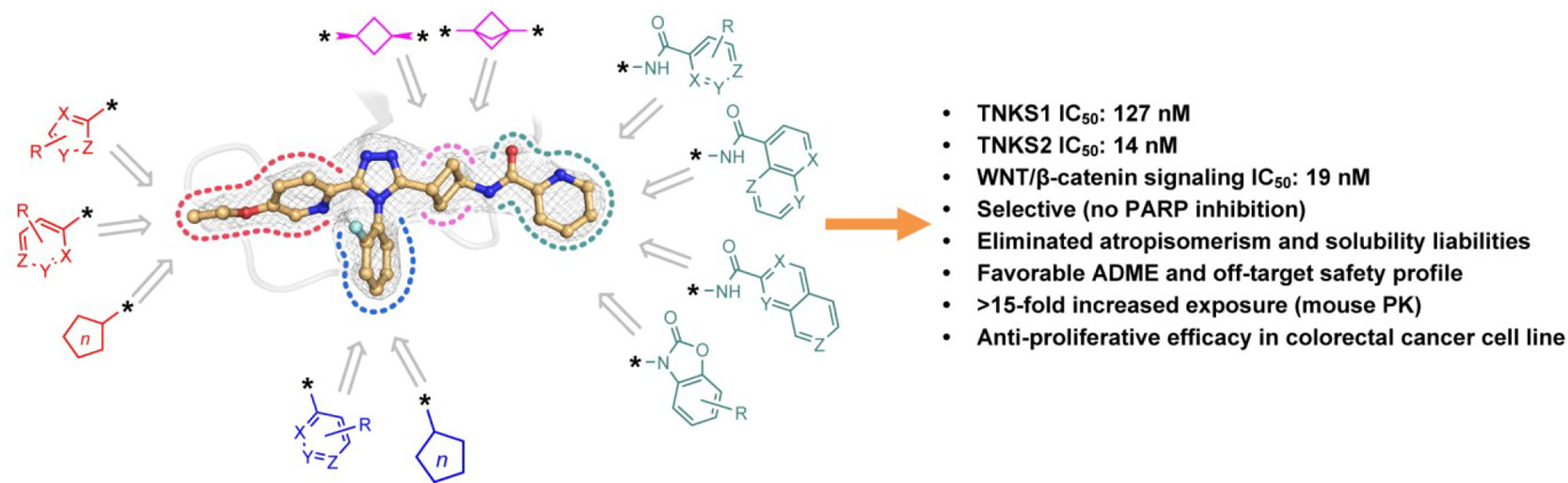
The Krauss group have previously developed tankyrase inhibitors bearing a 1,2,4-triazole moiety and binding predominantly to the adenosine binding site of the tankyrase catalytic domain. Here we describe a systematic structure-guided lead optimization approach of these tankyrase inhibitors. The central 1,2,4-triazole template and trans-cyclobutyl linker of the lead compound 1 were left unchanged, while side-group East, West, and South moieties were altered by introducing different building blocks defined as point mutations. The systematic study provided a novel series of compounds reaching picomolar IC50 inhibition in WNT/β-catenin signaling cellular reporter assay. The novel optimized lead shows a favorable ADME profile, including improved Caco-2 permeability and oral bioavailability in mice, and exhibits antiproliferative efficacy in the colon cancer cell line COLO 320DM in vitro. The group is currently advancing the tankyrase inhibition program to IND.
The work was published in the Journal of Medicinal Chemistry
Read the article here: